faStep® COVID-19 IgG/IgM Rapid Test Device
(Whole Blood/Serum/Plasma)(20 Test Box)
faStep® COVID-19 IgG/IgM Rapid Test Device (Whole Blood/Serum/Plasma) clinical trials were performed at two sites in China from February 2020 to March 2020. These clinical trials were aimed to evaluate the performance of COVID-19 IgG/IgM Rapid Test Device (Whole Blood/Serum/Plasma) by comparing with PCR.
Description
Highly accurate, easy to use rapid test kit provides results in 15 minutes
Includes:
- 20 tests/kit
- Disposable pipettes
- Sterile safety lancets
- Alcohol prep pads
- Buffer
- Package Insert
Intended use:
- Rapid lateral flow chromatographic immunoassay for qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood (sodium EDTA), serum, plasma (sodium EDTA) and fingerstick whole blood
- Identify individuals with an adaptive immune response to SARS-CoV-2
Limited use:
Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a
Note:
- Device should not be used to diagnose acute SARS-CoV-2 infection
- FDA, EUA, CLIA Waived authorization- fingerstick blood samples can now be tested in POC (Point of Care) setting such as doctor's offices, hospitals, urgent care centers and emergency rooms rather than having to be sent to a central lab for testing under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
The virus is thought to spread mainly from person-to-person.
- Between people who are in close contact with one another (within about 6 feet).
- Via respiratory droplets produced when an infected person coughs or sneezes.
- These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs.
Test Procedure
Allow the test device, specimen, buffer, and/or controls to reach room temperature (15-30°C) prior to
testing.
- Bring the pouch to room temperature before opening. Remove the test device from the sealed pouch
and use it as soon as possible.
- Place the test device on a clean and level surface.
Note:There should be a blue line in the control region (next to “C”), discard the device if there is no blue line.
- Label the test with patient or control identification.
- Add the specimens.
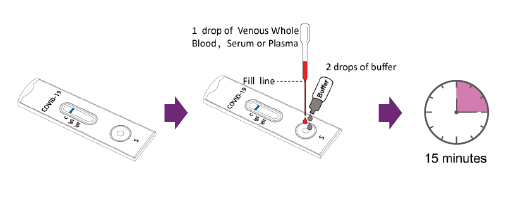
For Venous Whole Blood Specimens, Serum or Plasma Specimens
a) Using the provided disposable pipette, draw the specimen above the fill line (avoid the specimen entering the bubble of disposable pipette) and transfer one drop of the specimen into the specimen
well of the test device, then add 2 drops of buffer and start the timer. Adding more or less drops of
specimen may lead to incorrect results. Adding 1 drop of buffer or more than 4 drops of buffer
may lead to incorrect results.
For Fingerstick Whole Blood
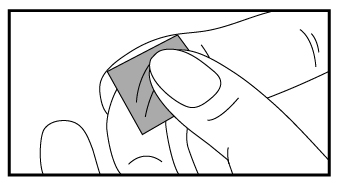
a) Clean the puncture site with the alcohol prep pad provided
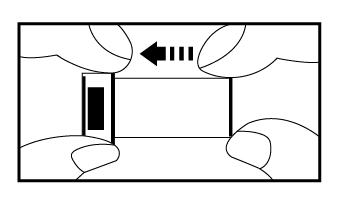
b) Carefully remove the cap from the safety lancet. Push the safety lancet firmly against the puncture
site until it pricks the finger.
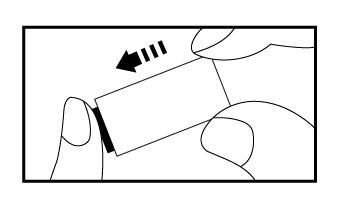
c) Using the provided disposable pipette, draw the specimen above the fill line(avoid the
specimenentering the bubble of disposable pipette) and transfer one drop (equivalent to 10μL) of
the specimen into the specimen well of the test device, then add 2 drops of buffer and start the
timer. Adding more or less drops of specimen may lead to incorrect results. Adding 1 drop of
buffer or more than 4 drops of buffer may lead to incorrect results.
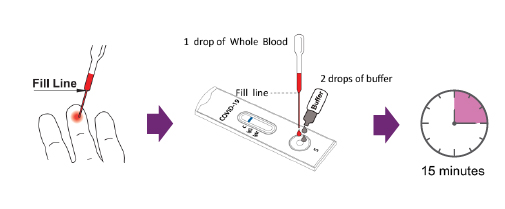
- 5. Wait for the blue line change to red line,read results at 15 minutes.
Note: Do not read results earlier than 15 minutes or after 30 minutes
Specimens can also be applied using a micropipette.
Result Interpretation
For Venous Whole Blood Specimens, Serum or Plasma Specimens
The
faStep® COVID-19 IgG/IgM Rapid Test Device detects anti-SARS-CoV-2 IgG/IgM antibody through visual interpretation of color development.
Anti-human IgG and anti-human IgM are used to detect specific antibodies in the human whole blood, serum, or plasma specimen. When specimen is added to the sample well, specific IgM and/or IgG antibodies, if present, will bind to the SARS-CoV-2 antigens conjugated to colored particles on the conjugate pad.
As the specimen migrates along the strip by capillary action and interacts with reagents on the membrane, the complex will be captured by anti-human IgM and/or anti-human IgG antibodies immobilized on the test region(s). Excess colored particle are captured at the internal control region.
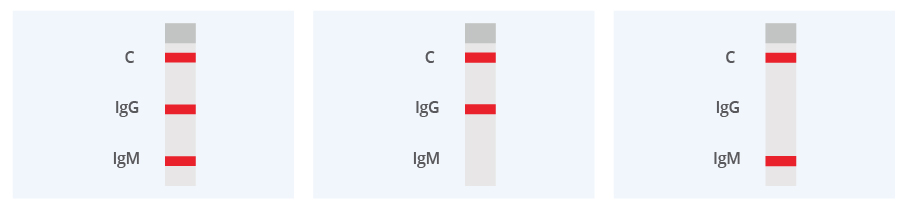
For faStep® COVID-19 IgG/IgM Test:
|
Positive
|
Positive
|
Positive
|
|
IgM and IgG Positive: The colored line in the control region (C) changes from blue to red, and two colored lines should appear in IgG and IgM test regions. The color intensities of the lines do not have to match. The result is positive for IgM and IgG antibodies.
|
IgG Positive: The colored line in the control region (C) changes from blue to red, and a colored lines appear in IgG test region. The result is positive for COVID-19 virus specific IgG antibodies.
|
IgM Positive: The colored line in the control region (C) changes from blue to red, and a colored lines appear in IgM test region. The result is positive for COVID-19 virus specific IgM antibodies.
|
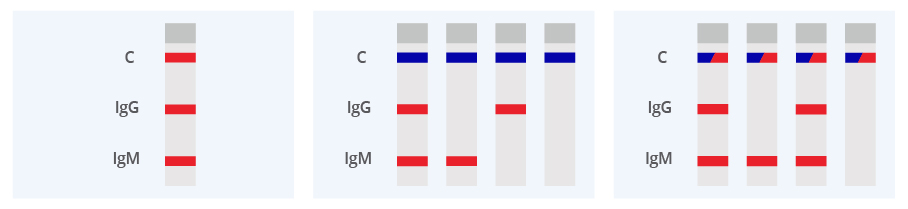
|
Negative
|
Invalid
|
|
Negative: The colored line in the control region (C) changes from blue to red. No line appear in IgM test region. The result is positive for COVID-19 virus specific IgM antibodies.
|
Invalid: Control line (C) is still completely or partially blue, and fails to completely change from blue to red. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
|
Note: The color intensity in the test region may vary depending on the concentration of analytes present in the specimen. Therefore, any shade of color in the test region should be considered positive. Note that this is a qualitative test only, and cannot determine the concentration of analytes in the specimen.
Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
Warning: The false positive, false negative, or invalid results may occur if the test is interpreted outside of the interpretation window.
The presence of a red band(s) on the test region(s) indicates a positive result for the particular IgG and/or IgM antibodies, while its absence indicates a negative result. A red band at the control region (C) serves as a procedural control, indicating that membrane wicking is working.
Product Performance Data
|
Antibody
|
Performance Measure
|
Estimate of Performance
|
95% Confidence Interval
|
|
LgM
|
Sensitivity
|
93.7% (74/79)
|
(86.0%; 97.3%)
|
|
LgM
|
Specifity
|
99.1% (225/227)
|
(98.6%; 99.8%)
|
|
LgG
|
Sensitivity
|
98.8% (82/83)
|
(93.5%; 98.8%)
|
|
LgG
|
Specificity
|
98.7% (224/227)
|
(96.2%; 99.5%)
|
‾‾‾
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection
of proteins from SARS-CoV-2, not for any other viruses or pathogens; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.