What is Oxycodone (Percocet)?
Oxycodone is known as Oxycontin and Roxicodone. It is an ingredient of Percodan, Percocet, Roxicet and Tylox. Oxycodone is a semi-synthetic opiates derived from opium. Like other opiates, Oxycodone is characterized by its analgesic properties. The tendency for users to form a physical dependency and develop tolerance increases with extended use. Oxycodone is usually administered in combination with non-opiate analgesics such as acetaminophen and salicylates for the relief of moderate to severe pain. Oxycodone is a central nervous system depressant that may cause drowsiness, dizziness, lethargy, weakness and confusion. Toxicity in an overdose of Oxycodone can lead to stupor, coma, muscle flaccidity, severe respiratory depression, hypotension, and cardiac arrest. Oxycodone is metabolized by N- and O-demethylation. One of the metabolites, oxymorphone, is a potent narcotic analgesic, while the other, noroxycodone, is relatively inactive. Between 33 to 61% of a single dose of Oxycodone is excreted in a 24 hour urine collection and consists of 13-19% free Oxycodone, 7-29% glucuronide conjugated Oxycodone, 13-14% glucuronide conjugated oxymorphone and an unknown amount of noroxycodone. The detection time window of Oxycodone is 1-3 days following use.
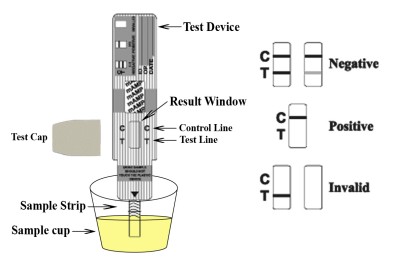
Oxycodone (Percocet) Drug Test Procedure
To use a OXY drug test kit your will need a *
disposable collection cup and follow this procedure:
- Collect urine from the donor in a disposable collection cup.
- If the collection cup has a temperature strip read it and make sure is between temperature range.
- Remove the bottom cap from the OXY drug test card and submerge the drug test strips in the urine.
- AVOID passing the arrow when submerging the single drug test card since this can flood the test and invalidate the drug test.
- Once all the lines in the C (control) area appear you can read the bottom lines in T (test) area.
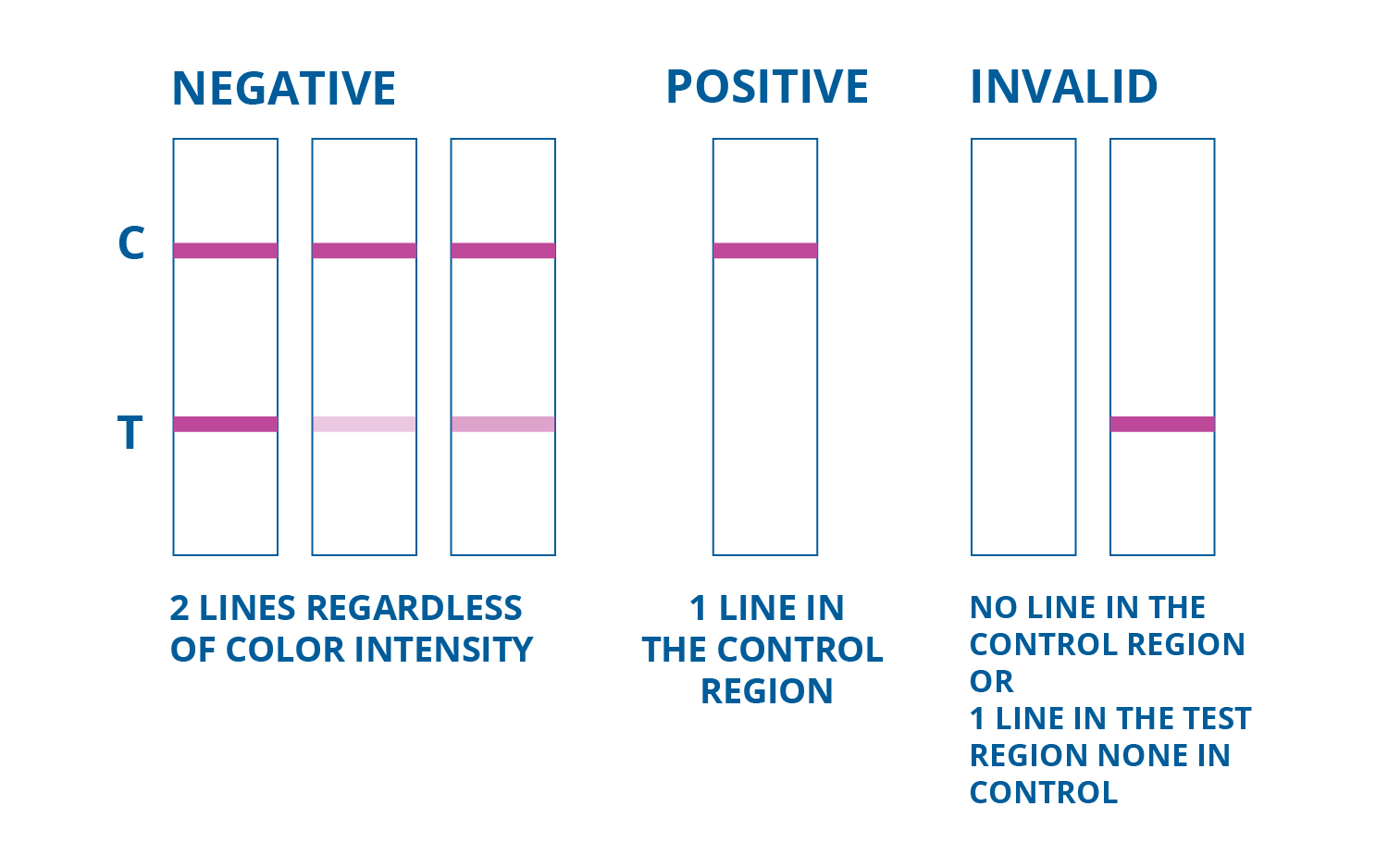
- To interpret results in the single drug test card follow the image below
CLIA WAIVED Oxycodone Single Drug Test
---
Note: CLIA WAIVED Single Drug Test Cards
Our Single Panel Drug Test Cards are CLIA Waived, which means they meet the standards set by the Clinical Laboratory Improvement Amendments (CLIA) for use in non-laboratory settings. These tests are approved for point-of-care use, ensuring high accuracy and reliability in workplace, clinical, and home environments. By choosing CLIA Waived products, you are selecting tests that adhere to strict regulatory standards while simplifying the testing process for any user.
* Disposable Collection Cup Sold Separately