DPP® HIV 1/2 Assay
Oral and Blood Assay Test Procedure for In Vitro diagnostic use
Rapid Point-Of-Care testing for antibodies of HIV-1 and HIV-2
Oral Fluid and Whole Blood Test Procedure
CLIA Complexity WAIVED for oral fluid, fingerstick whole blood, and venous whole blood
- Before performing testing, all operators MUST read and become familiar with Universal Precautions for prevention of transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other blood borne pathogens in Heath-Care Settings.
- These instructions are not only a Reference Guide for use in CLIA waived settings. Read the complete procedure, including the QC procedure, before performing the test.
- Laboratories with a Certificate of Waiver must follow the manufacturer's instructions for performing the test. Any modification by the user(s) to the manufacturer's test procedures will result in the test no longer meeting the requirements for waived classification.
External Quality Control:
A Chembio DPP® HIV 1/2 Rapid Test Control Pack is available separately for use with the Chembio DPP® HIV 1/2 Assay. The HIV Controls are used to verify the operator's ability to properly perform the test and interpret the results. Run the controls as described in the Test Procedure section for a whole blood sample and follow the Interpretation of Results section below.
We recommend running controls:
- with each new operator
- with each new device lot
- with each new shipment of devices
- if improper device storage is suspected and
- at periodic intervals
It is the responsibility of each facility using the Chembio DPP® HIV 1/2 Assay to establish an adequate quality assurance program to ensure the performance of the device in their environment and condition of use.
DPP® HIV 1/2 Test Procedure:
Adequate Lighting Required
Important: Follow the oral fluid sample collection instructions carefully, as not doing so may produce incorrect results.
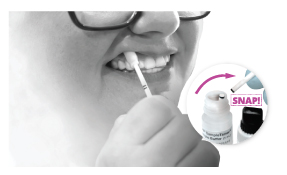
Step 1
Step 1 Option 1
Swab outer gums for 30 seconds
|
Step 1 Option 2
Use loop to obtain sample
|
|
|
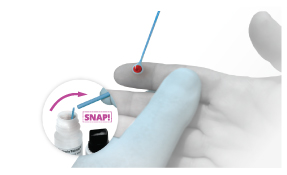
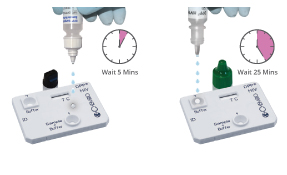
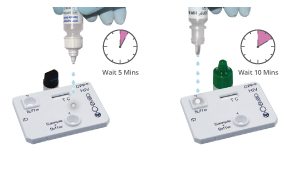
Step 2
Step 2 Option 1
Oral Fluid Test
|
Step 2 Option 2
Blood or Finger-stick Test
|
|
|
- 2 drops sample
+ Buffer to well 1
- 4 drops running
buffer to well 2
|
- 2 drops sample
+ Buffer to well 1
- 4 drops running
buffer to well 2
|
Step 3
Results Interpretations
Interpretation of Results: Oral Fluid
HIV Non-reactive (-)
One pink/purple line in the CONTROL (C) area, with no line in the Test area, is a Nonreactive result. A nonreactive result at 40 minutes means that HIV antibodies were not detected.
The test result is NEGATIVE for HIV antibodies. However,
this does not exclude possible infection with HIV.
HIV Reactive (+)
A pink/purple line in the CONTROL (C) area, with a line in the Test area, is a Reactive result. If ANY VISIBLE LINE appears in the TEST area and in the CONTROL area, no matter how faint, the result is REACTIVE. A reactive result between 25 and 40 minutes means that HIV antibodies were detected.
A REACTIVE test result means that HIV antibodies were detected in the specimen. The test result is Preliminary POSITIVE for HIV antibodies.
Invalid Results
If no control line appears, results are invalid whether or not a test line is present. An INVALID test result means there was a problem running the test. The problem could be due to the specimen, the test device or the procedure.
An INVALID test result cannot be interpreted. An INVALID test should be repeated with a new device.
Interpretation of Results: Finger Stick or Venous Whole Blood
HIV Non-reactive (-)
One pink/purple line in the CONTROL (C) area, with no line in the Test area, is a Nonreactive result. A nonreactive result between 10 and 25 minutes means that HIV antibodies were not detected.
The test result is Negative for HIV antibodies. However,
this does not exclude possible infection with HIV.
HIV Reactive (+)
One pink/purple line in the CONTROL (C) area, with a line in the Test area, is a Reactive result. If ANY VISIBLE LINE appears in the TEST area and in the CONTROL area, no matter how faint, the result is REACTIVE. A reactive result between 10 and 25 minutes means that HIV antibodies were detected.
A REACTIVE test result means that HIV antibodies were detected in the specimen. The test result is Preliminary POSITIVE for HIV antibodies.
Invalid Results
If no control line appears, results are invalid whether or not a test line is present. An INVALID test result means there was a problem running the test. The problem could be due to the specimen, the test device or the procedure.
Note: An INVALID test result cannot be interpreted. An INVALID test should be repeated with a new device.
Product Performance
|
Sample Type
|
Sensitivity
|
Specificity
|
|
Oral Fluid
|
98.8%
|
99.9%
|
|
Finger-stick
|
99.8%
|
100%
|
|
Whole Blood
|
99.9%
|
99.9%
|
|
Plasma
|
99.9%
|
99.9%
|
|
Serum
|
99.9%
|
99.9%
|